Vector Development
Therapeutic proteins are biologically derived macromolecules, most commonly produced using recombinant DNA technology in microbial or mammalian expression systems. These therapeutic proteins are used to prevent, treat, or cure a wide spectrum of diseases. The category for therapeutic proteins includes monoclonal antibodies, cytokines, growth factors, hormones, and enzymes, etc.
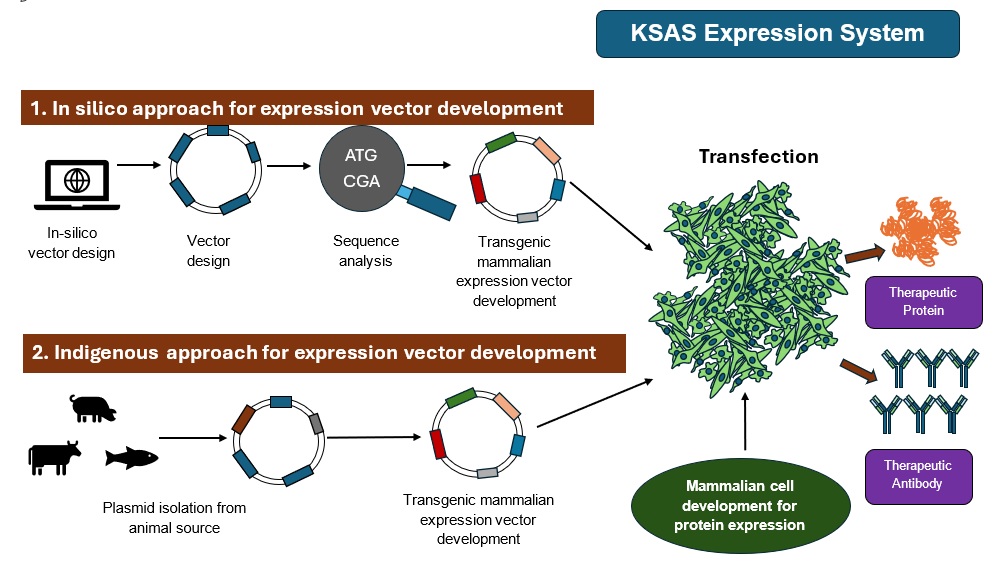
At KSAS, through a combination of in silico molecular design and indigenous biotechnological approaches, we have developed customized expression vectors optimized for high-level production of therapeutic proteins.
To achieve robust and scalable expression, a specialized mammalian expression platform is required, as mammalian cells are uniquely capable of performing post-translational modifications that are essential for the biological activity, stability, and therapeutic efficacy of recombinant proteins. In line with this, we are in the process of developing our own mammalian cell line, engineered for high productivity, genetic stability, and enhanced secretion ability.
Part 1
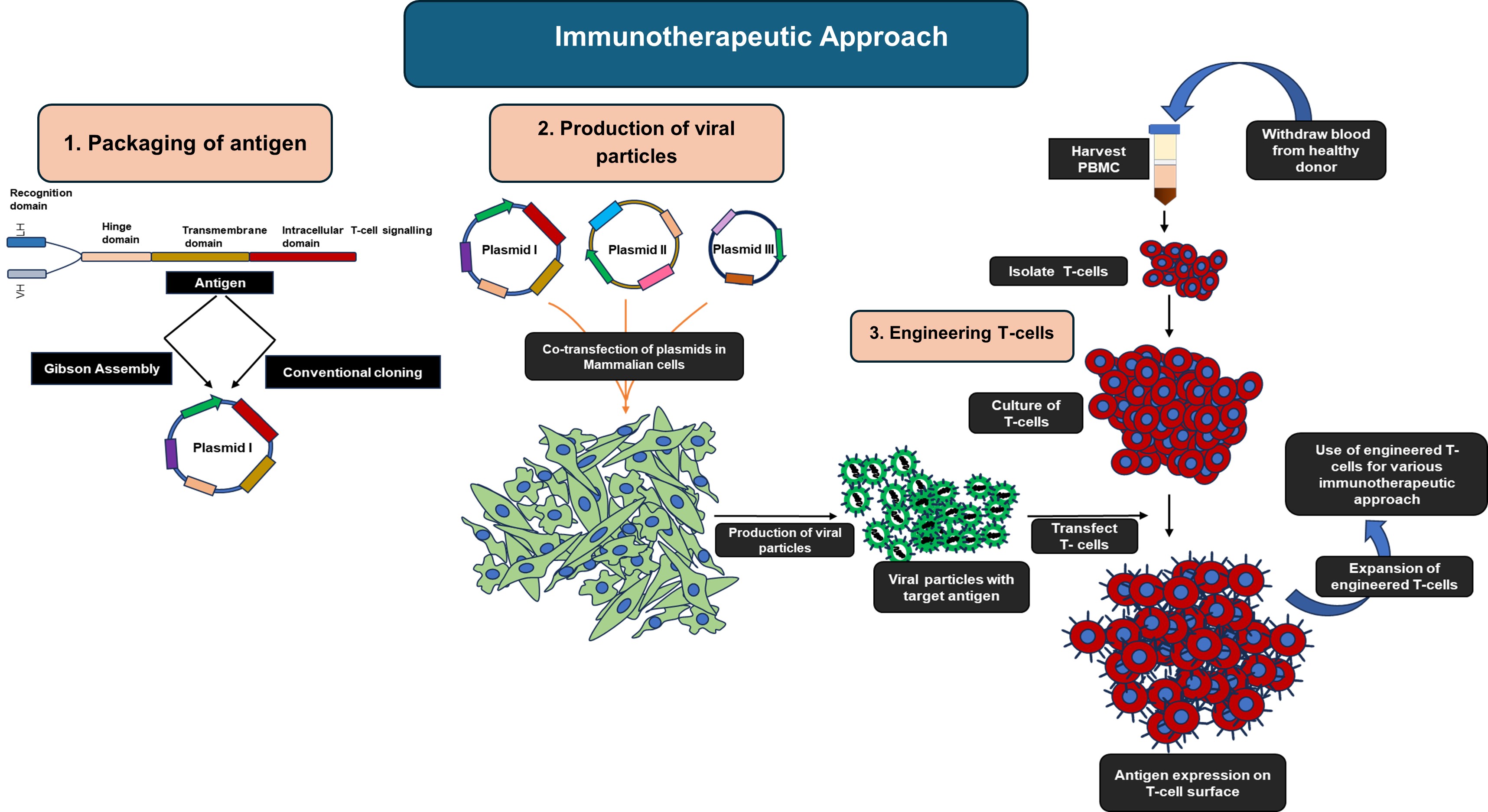
Cancer continues to be one of the leading causes of mortality worldwide. Chimeric Antigen Receptor T-cell (CAR-T) Therapy is one of the most life-changing developments in modern medicine to treat certain types of cancer. Conventional approaches such as chemotherapy or radiotherapy kill both malignant and healthy cells; However, CAR-T therapy harnesses the body’s own immune system to selectively kill cancerous cells.
In this approach, T-lymphocytes are engineered ex vivo using viral or non-viral delivery systems to introduce CAR, which contains an extracellular antigen-recognition domain, a transmembrane domain, and an intracellular signaling domain. T cells, developed by this process, can recognize tumor-associated antigens and mount a potent cytotoxic response. These engineered T-cells are expanded in vitro to use for different immunotherapeutic approaches.
At KSAS, we seek to develop CAR-T technology not only for cancer but also against autoreactive B-lymphocytes, which are central drivers in many autoimmune diseases. Our aim is to achieve a long-lasting disease remission without suppressing the immune system. This strategy offers a significant advantage over current immunosuppressive regimens, which often result in systemic toxicity and increased infection risk. Our long-term vision is to develop immunotherapies not only for oncology but also for immunological and inflammatory disorders, to reshape personalized medicine, and enable safer and disease-specific interventions.
Part 2
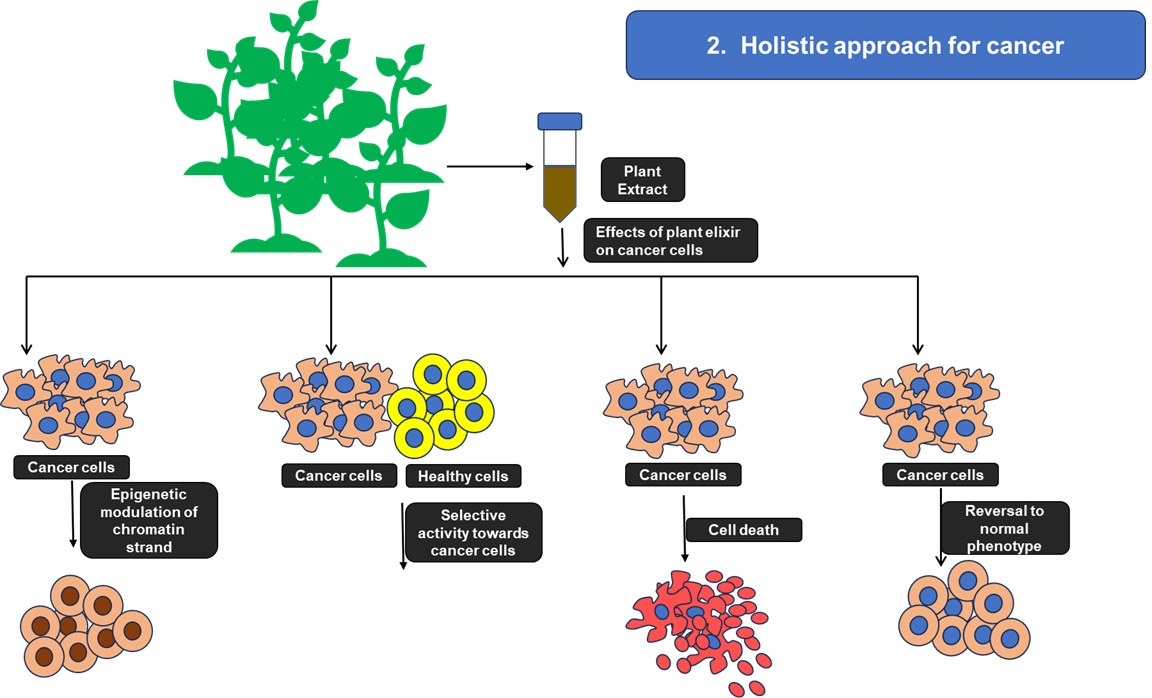
Conventional cancer treatments, although effective in many cases, are associated with severe adverse effects due to their lack of specificity, killing malignant cells and also rapidly dividing healthy cells such as those in the gastrointestinal tract, bone marrow, and hair follicles. This underscores the urgent need for novel therapeutic strategies with improved selectivity and reduced systemic toxicity.
A holistic and plant-derived therapeutic approach has emerged as a promising alternative. Various medicinal plants, including Ocimum sanctum, Azadirachta indica, and Curcuma longa, contain phytochemicals such as flavonoids, terpenoids, alkaloids, and polyphenols, which show potent anticancer activity. These bioactive compounds have been shown to induce apoptosis, modulate cell cycle regulators, suppress pro-tumorigenic signaling cascades, inhibit angiogenesis and metastasis, and enhance antioxidant defense mechanisms. Interestingly, certain plant extracts have shown selective cytotoxicity towards cancer cells while avoiding normal cells. Additionally, some of these potential compounds have been reported to reprogram epigenetic modifications and alter gene expression, and thereby revert malignant cells to a more differentiated or normal-like phenotype. This ability to induce “phenotypic reversion” highlights a novel dimension of phytotherapy in oncology.
At KSAS, our research focuses on exploring extracts from various parts of medicinal plants, including leaves, roots, seeds, and bark, to assess their potential anti-cancer properties. We aim to elucidate molecular mechanisms through which these phytoconstituents can restore normal cellular functions. By integrating plant-based therapeutics with modern molecular biology approaches, we envision the development of safer and more effective anticancer treatments that complement or surpass conventional chemotherapies.
Neuronal Regeneration and Degeneration
Part 1
Regeneration of the central nervous system (CNS) remains one of the longstanding and most complex challenges in neuroscience, particularly in the context of restoring functional neurons after injury or disease. Effective CNS regeneration requires coordinated regulation of two critical processes: a) Activation of endogenous mechanisms that promote functional neurite outgrowth, and b) Modulation of the exogenous inhibitory environment, which often impedes axonal regeneration and neuronal repair.
At KSAS, we are actively working to develop a comprehensive therapeutic strategy that addresses both of these barriers. Our research focuses on designing two complementary approaches: one to stimulate intrinsic neuronal growth potential, and another to neutralize or reprogram the inhibitory extracellular environment that surrounds damaged neural tissue.
Furthermore, we are investigating how these two strategies can be applied synergistically to enhance neural regeneration in a more effective and sustained manner. Our aim is to create an integrated treatment paradigm that promotes both structural and functional recovery of the CNS, paving the way for novel regenerative therapies in neurotrauma and neurodegenerative diseases.
We are utilizing spinal cord injury (SCI) as a model system in our research on CNS regeneration. Traumatic SCI has long been recognized as a major global health concern, placing a substantial burden on health care systems worldwide due to its high costs, complex care needs, and long-term impact on patients. SCI is a severe medical condition that results in extensive neuronal damage and chronic functional impairments, often leading to permanent disabilities and significantly diminishing the quality of life for affected individuals.
According to the Global Burden of Disease Study 2021 (GBD 2021), the world sees approximately 5,74,500 new SCI cases every year, with approximately 15.4 million prevalent cases. The disability burden (measured in years lived with disability (YLDs)) is around 4.6 million. Although age-standardized incidence rates have declined (1990-2021) and are projected to decline through 2050, the absolute burden of SCI is increasing due to population growth and aging demographics. These figures highlight an urgent need for enhanced strategies in prevention, acute care, long-term rehabilitation, and social reintegration.
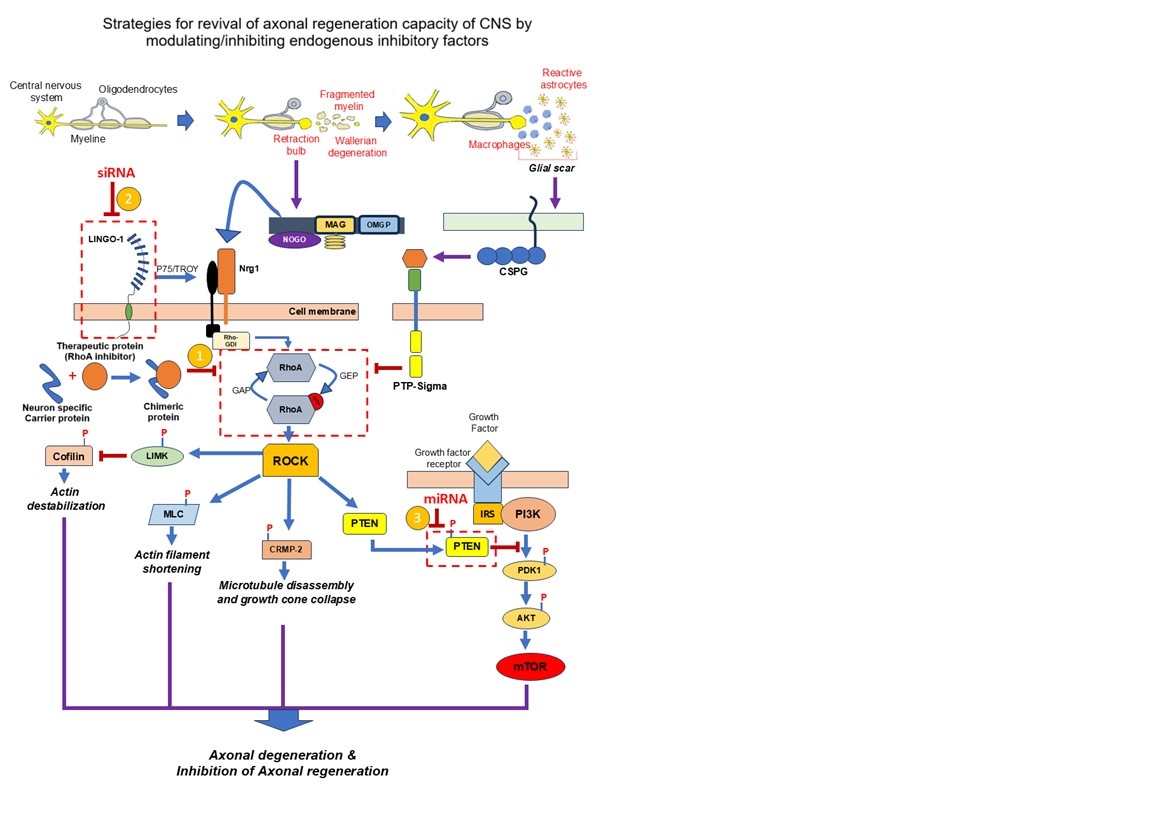
In CNS, the axonal injury is typically followed by axonal disintegration and demyelination, which initiate a cascade of cellular and molecular responses. Among these, reactive astrocytes and macrophages are recruited to the injury sites, forming a dense, protective structure known as the Glial Scar. While the glial scar plays several protective functions, it also acts as a physical and biochemical barrier to axonal regeneration. One of the primary inhibitory mechanisms is the activation of RhoA signalling pathways through overexpression of RhoA in the injured neurons, which inhibits cytoskeletal dynamics essential for axonal extension. Additionally, myelin debris at the injury site exposes a range of inhibitory molecules like Nogo, oligodendrocyte-myelin glycoprotein (OMgp), and myelin-associated glycoprotein (MAG). These molecules interact with receptors such as NogoR (a receptor protein). and p75 (a neurotrophin receptor) further contributes to the activation of RhoA signalling and suppression of functional axonal generation. Given that RhoA signalling is a central convergence point for multiple inhibitory pathways and can be considered as the potential target for the axonal regeneration therapy.
We, here at KSAS, along with our parent organization INADS (Institute of Advanced Sciences), USA, are actively developing therapeutic strategies targeting RhoA signalling to promote the regeneration of functional neurons following SCI. While inhibition of RhoA signaling is a critical step toward overcoming the inhibitory environment of the injured CNS, effective therapeutic intervention also requires precise targeting of these molecules to the site of injury.
To address this challenge, we are pursuing the development of advanced delivery systems, including botulinum heavy chain–based carriers and viral vector–based platforms, to ensure site-specific delivery of therapeutic agents. We have successfully engineered and tested chimeric fusion proteins that combine therapeutic functionality with targeted delivery capabilities. These constructs have demonstrated the potential to inhibit RhoA activity via ADP-ribosylation, a key mechanism for facilitating axonal regeneration.
In parallel, we are advancing the development of recombinant adeno-associated viruses (rAAV) and functionalized liposomes as novel delivery systems designed for the selective targeting of neurons. These platforms aim to modulate gene expression critical for remyelination and the generation of functional neurons, further supporting neural repair and recovery.
Our integrated approach—combining targeted molecular therapy with innovative delivery technologies—positions KSAS and INADS at the forefront of next-generation treatments for CNS injuries.
Part 2
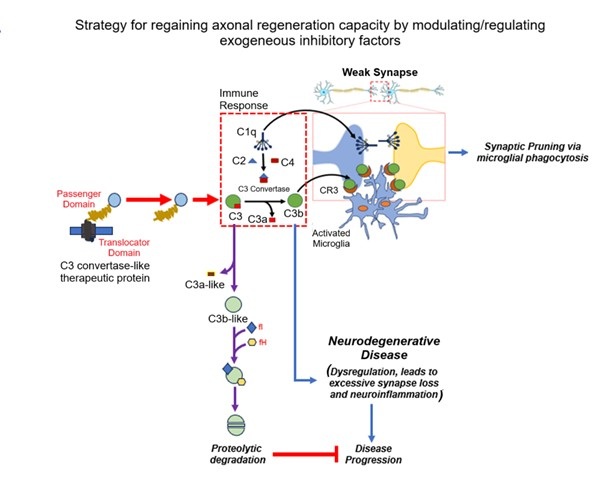
One of the key factors in the neuroinflammatory response is the C3 complement component, which is a central protein in the immune system's complement cascade. During normal brain development, the activation of the classical pathway leads to C1q expression, causing cleavage of C4 and C2 and resulting in expression of C3, which tags weak or unhealthy synapses. The C3-tagged synapses are recognized by microglia. Microglia carry CR3 receptors, which bind to the C3 and initiate the process of phagocytosis (synaptic pruning).
The neurodegenerative diseases like Alzheimer’s disease (AD), Parkinson's disease (PD), and multiple sclerosis (MS) lead to an aggressive inflammatory response and axonal degeneration in the CNS. In neurodegenerative disease conditions, C3 is significantly upregulated and initiates unwanted synaptic pruning, which causes excessive synapse loss and cognitive decline. Therefore, controlling C3 expression is a promising avenue to combat neurodegenerative diseases, especially if interventions occur early in the disease process.
We, here at KSAS, along with our parent organization, INADS (Institute of Advanced Sciences), USA, are developing therapeutic strategies for controlling the aggressive inflammatory responses following the neurodegenerative disease by inhibiting the overexpression of the C3 component of the complement system. While inhibition of the overexpression of C3 complement component is a critical step toward overcoming the external inhibitory factors for axonal regeneration, effective therapeutic intervention also requires precise targeting of these therapeutic molecules to the diseased site. Therefore, we are simultaneously focusing on the development of advanced delivery systems, including botulinum heavy chain–based carriers and viral vector–based platforms, to ensure site-specific delivery of therapeutic agents.
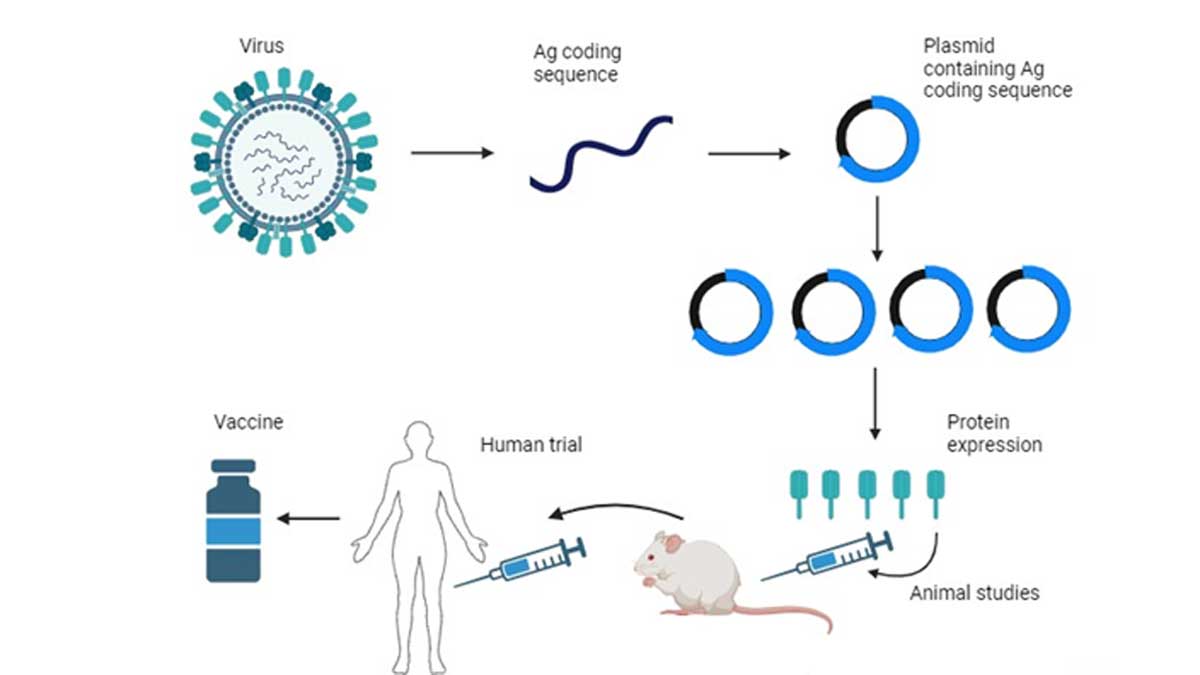
Vaccine is the most efficient safeguard against a viral infection. Though nucleic acid-based vaccines are most preferred as of now, it has risk associated with unwanted side effects and r genome integration. Protein-based vaccines are the safest vaccine. Protein vaccines are comparatively safer tools because of definite half life inside a living organism that doesn’t poses a permanent genome integration risk and generates a higher antigenic response compared to nucleic acid-based vaccines. As a safer and better efficient alternative, KSAS team, along with INADS USA, is working on protein-based vaccines for several existing viral infections.
Therapeutic antibodies represent a powerful class of biopharmaceuticals that are widely used for the treatment of cancer, autoimmune disorders, and inflammatory diseases, as well as for targeted drug delivery to specific antigens. The majority of these therapeutic molecules are monoclonal antibodies (mAbs), which are designed to recognize and bind with high specificity to a single epitope on a target antigen. Upon binding, these antibodies can either block critical signaling pathways to inhibit cancer cell proliferation or activate immune-mediated mechanisms to eliminate diseased cells.
The mechanism of action of therapeutic antibodies is fundamentally distinct from that of conventional small-molecule drugs, even when both target the same antigen. While small molecules typically function by inhibiting or modulating intracellular pathways, antibodies exert their effects through extracellular binding events that lead to receptor blockade, immune effector recruitment, or the targeted delivery of therapeutic payloads. Compared to small molecules, monoclonal antibodies offer several advantages, including exceptionally high target affinity, remarkable specificity, and generally low immunogenicity, making them safer and more effective for long-term therapeutic use.
At KSAS, we employ a multifaceted strategy to develop next-generation therapeutic antibodies:
a) Antibody engineering – Modifying different regions of the antibody structure (e.g., variable regions, Fc domains) to enhance binding, stability, and effector functions for a range of therapeutic targets.
b) Improving specificity – Developing innovative strategies to minimize off-target interactions, thereby improving therapeutic precision and reducing adverse effects.
c) Diversifying antibody formats – Exploring and developing various types of antibody constructs, including humanized antibodies, bispecific antibodies, and antibody fragments, to address diverse clinical needs and improve therapeutic outcomes.
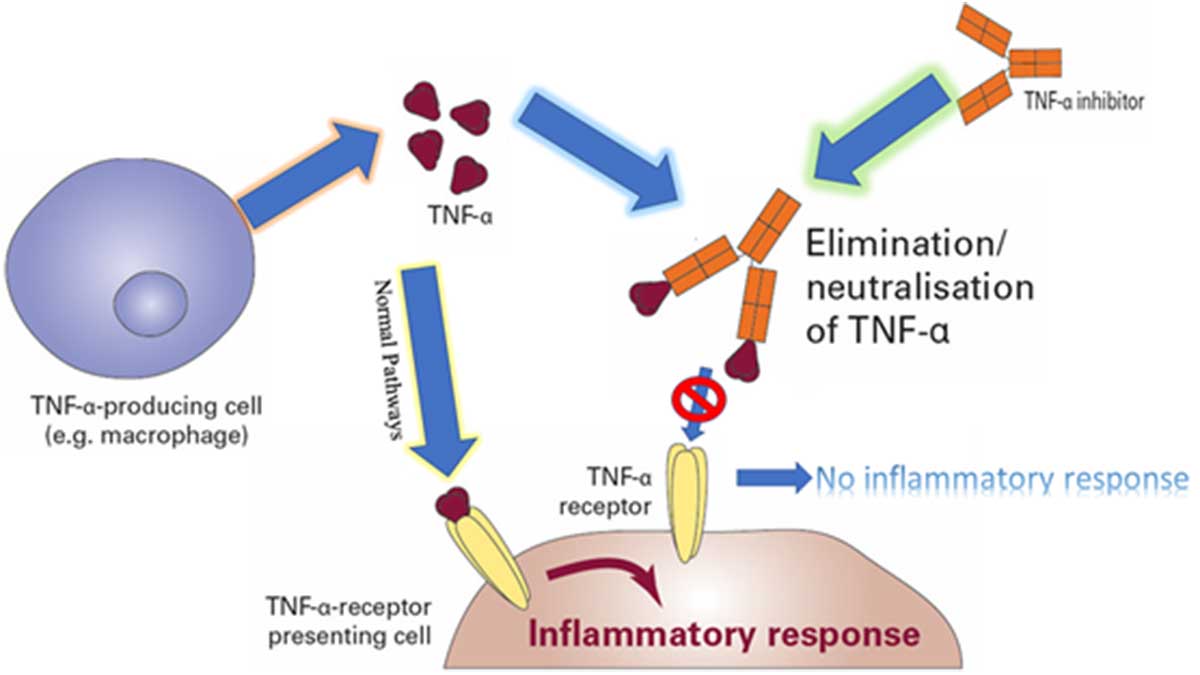
An illustration of inflammatory response of TNF-a.